Oxygen Scavenger
The Oxygen Corrosion Problems – Complete Solution
The problem of Boiler Corrosion is extremely expensive. The amount of money one has to pay that arises due to repair work of metal, loss of energy and valuable time results in millions of dollars. But, one can easily prevent the problem of corrosion without incurring a lot of expense.
The corrosion that occurs in boilers is due to the dissolved oxygen that is present in the steam which is being generated along with the feed water. The solution to this problem lies in how one can prevent the oxygen from causing any damage. Majority of the systems use mechanical oxygen removal which is present in deaerators. Chemical Reducing Agents are also added to this.
The use of these agents help to combine with the dissolved oxygen and form products that are harmless. That is why they are known as the oxygen scavengers.
Oxygen in boiler water
We know that oxygen plays a role in corrosion process of boiler and metals. The dissolved gas reaches steam generator through water. Since oxygen is the damaging factor, we must remove it. In this case, we may use reducing agents and mechanical removal process.
Oxygen over metal
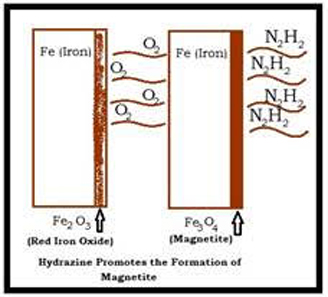
Magnetite is formed for the reaction of hot ionized water and steel. The thin magnetite layer on the surface of boiler works as the best water-resistant barrier of corrosive components and metal.
However, while there is dissolved gas (like oxygen), it will cause an impact on the barrier. The reaction of magnetite and oxygen results in an irregularity to the barrier. This impact of oxygen will form rust (chemical formula- Fe203).
Hydrazine plays a vital role in the protection of boiler steel simply by controlling the rust.
N2H4 + 6Fe2O3![]() 4Fe3O4 + 2H2O + N2
4Fe3O4 + 2H2O + N2
The main thing that is essential is some amount of hydrazine. Theatrically, magnetite is also produced due to sodium sulfite.
Na2SO4 + 3Fe2O3![]() 2Fe3O4 + Na2SO4
2Fe3O4 + Na2SO4
Various chemical trials have proved that the temperature requirement for this type of reaction is minimum 430°F. However, at about 250°F, hydrazine starts reduce the corrosion. This is also intended to create magnetite on steel at a lower temperature, like 120°F.
Corrosion Reaction
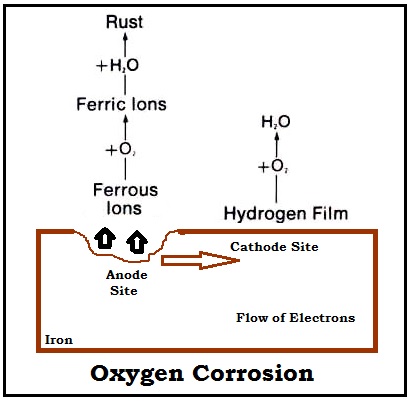
All the battery users are familiar to the electrochemical reaction, like corrosion. While the surface of any metal is not regular or consistent, it can create anodic site. Water is chosen as the electrolyte, while metal becomes electrons conductor. However, there will be no current flow through the polarized cathodic site.
When water reacts with steel, it produces hydrogen. The cathode site gets a thin film while there is no oxygen. Oxygen, in dissolved form, gets blended with hydrogen in water. Current can start flowing due to the de-polarized cathodic site. While this current of corrosion begins to flow, the ions of iron become combined with solution. We can find the presence of ions in this solution as a type of dissolved components. Finally, they get blended with the gas (oxygen) for the production of iron oxides. These oxides remain as residue on a metal, and that is what we call as rust. With the elimination of the dissolved gas, hydrogen layer restores its position and polarize cathode site.
Thus, by avoiding oxygen gas from water, we will be able to keep away from corrosion. We can add Remov –OX to boiler water for oxygen removal purpose and for corrosion problem solution.
What is an oxygen scavenger
Dissolved oxygen gets blended with reducing agents to create harmless products that we call as oxygen scavengers. They are chemicals that we use for boiler water for the elimination of oxygen, left as residue.
Hydrazine can be used as a type of oxygen scavenger. Now, for comparing various products and for dealing with various concerns, we have presented some queries and the accurate answers that help anyone to make decision. We think that it will remove your concerns and solve your queries on Remov – Ox.
Why use an oxygen scavenger
Oxygen, in dissolved form, results in corrosion at an intense pressure and temperature, found in boiler. Thus, it is essential to remove oxygen.
What are the properties of good oxygen scavengers
Fastest reaction with oxygen, volatility, absence of solids and passivating steel object- these are the properties of oxygen scavengers.
